
Nymalize is the liquid nimodipine you know and trust for your patients with SAH1,2,4-6
The only RTU liquid nimodipine offering multiple dosing and administration options1
American Society of Health-System Pharmacists guidelines recommend the use of ready-to-use medications whenever possible to reduce situations requiring manipulations and prevent errors7
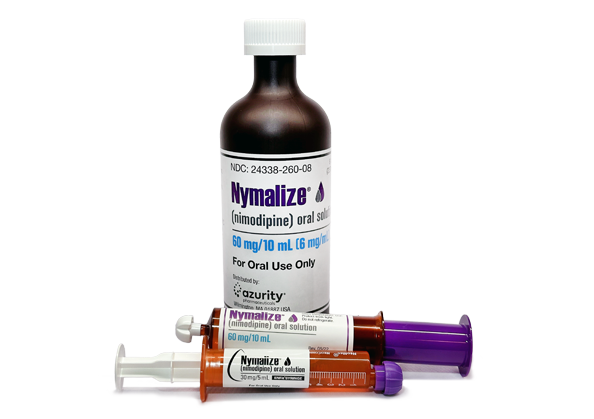
Nymalize has efficient and flexible RTU dosing options that include prefilled oral syringes, prefilled ENFit syringes, and bottles to meet the needs of patients, providers, and hospitals alike.1
About nimodipine: a trusted molecule since 19888
- Nimodipine reduces the incidence and severity of brain function loss in stroke patients.1 Nimodipine was originally only available as liquid-filled gel capsules, but most stroke patients have difficulty swallowing capsules.9 Dysphagia is a common occurrence after stroke, happening in ≥65% of stroke patients10
- Before Nymalize, the practice of extracting nimodipine from capsules was the only option6,9
- If the capsule cannot be swallowed, the contents of the capsule are extracted into a syringe using a needle and administered via a nasogastric tube9
Timeline of Nimodipine Advancement
19888
Nimodipine oral capsule
20102
FDA issues safety communication warning against intravenous use of nimodipine oral capsules
201311

Discontinued 202011
202012

NYMALIZE HAS BEEN REFORMULATED TO INCLUDE
Higher concentration (6 mg/mL) per dose1,13
50% reduction in liquid volume per dose1,13
44% less polyethylene glycol per 60 mg dose14,a
a6.6 grams polyethylene glycol/60 mg dose.
20241


When it comes to extracting nimodipine, the chance for errors is high2
For the standard dose of 60 mg (2 x 30 mg capsules every 4 hours), liquid nimodipine must be extracted from 12 capsules per day9
- Potential risk for needle sticks during the extraction process8
- Pharmacy is required to prepare extracted liquid nimodipine in advance or forces the ICU nurse to perform a bed-side extraction to ensure appropriate nimodipine treatment initiation per comprehensive stroke center (CSC) quality metrics4,15
There is a potential for ≈25% dose reduction with each 60 mg dose8
Capsule extraction is time-consuming and exposes patients to potentially lethal medication errors2,9
Pharmacy prepares extracted liquid nimodipine in advance
ICU nurse performs a bed-side extraction
Capsule Extraction Methods15
Between 1989 and 2013, 36 nimodipine-related medication errors were reported; 27 of these were associated with erroneous IV administration, and 5 of these cased resulted in death.2
Reducing risk to patients is a priority, using Nymalize provides precision and may reduce dosing and administration inaccuracy1
